SEDDS Technology
This is your Project Page. It's a great opportunity to help visitors understand the context and background of your latest work. Double click on the text box to start editing your content and make sure to add all the relevant details you want to share.
SEDDS Technology Research Lab
SEDDS (Self Emulsified Drug Delivery System) Technology Research Lab is the subsidiary research company of MTC Industries which was founded by Dr. Jimmy Wang, in 2001 on Long Island, New York. MTC owns the namesake brand “SEDDS”.
The research fellows of SEDDS Technology Research Lab are a group of experienced scientists from top pharmaceutical companies and universities in the United States. Our goal is to develop new formulations and new products to improve and increase the absorption and bio-availability of nutrition and screen new active compounds from wild natural source.

Research Focus
Soluble platform construction of insoluble and active phamaceutical & Nutraceutical molecules and industrialization

Project Background
In ten years, in the lead compound library, high solubility and high permeability, high solubility and low permeability, low solubility and high permeability compounds have been a hot research object. To improve the bioavailability of new drugs, the Lipinski Screen Five Principles are summarized based on the experience, namely, a molecular weight of less than 500 Dalton, the hydrogen bond acceptor number, no more than 10, the number of hydrogen bond donors of not more than 5, the lipid water partition coefficient (logP) is between -2 to 5. In the process of screening lead compounds, for the considerations of compounds bioavailability, most active compound molecules are abandoned after first round screening. Now the compound library resources (Lipinski drug screening five principles) is full developed, the compound whcih has good efficacy, but bad bioavailability (not comply with the Lipinski screen five principles) has become the gold mining to be developed for new drugs.

Present market on research topic
By the polymeric micelles technology of the solid dispersion, a series of compounds with good efficacy and insolubility were successfully developed into the listed drugs.
By the SEDDS technology of the solid dispersion, a series of compounds with good efficacy and insolubility were successfully developed into the listed drugs.



Technology Platform
Considering the factors of SEDDS technology:
(1) The classification of drug vehicles or nutrient molecules; (2) The SEDDS system classification, Surfactant, Cosurfactant, Solvent, Oil selection; (3) The preparation process of SEDDS.
The preparation process of SEDDS :
(1) The method of self assembly of the solvent evaporation. (2) Dialysis. (3) The evaporation method of emulsion and solvent (4) The chemical combination method.
Detection technology of SEDDS:
(1) Its distribution of the morphology, particle size. (2) The measure of entrapment efficiency and drug loading. (3) The extent of oxidized phospholipids (4) The provisions complying with the general principles of phamaceutics. (5) The stability of solution (pH, Temperature, Centrifugation, Test). (6) Zeta potential, Viscosity, Cloud Point, Dye Solubilization Test.
The new technology of s-SEDDS and its problems:
SEDDS has low stability, the irreversible separation of drug loading and lipid carrier system, big dose, difficultly portable dosage form. Therefore the solid -SEDDS is the developing hotspot at present, Examples of such problems include the following:
(1) Amount of solidifying excipients may affect the release of the drug.
(2) Nature of the excipients used may affect the drug absorption.
(3) Probability of irreversible phase separation on reconstitution.
(4) Clogging of spray nozzles due to oil content in spraydrying method.
(5) Degradation of drug during solidification process.
(6) Reduction in drug loading capacity.
(7) Difficulty in ensuring content uniformity.
(8) Probability of residual solvents used during granulation.
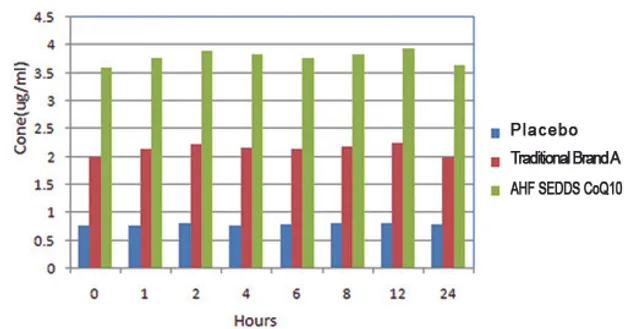
Clinical trial of the MTC research group:
In a recent double-blind clinical study, SEDDS
Coenzyme Q10 softgel has shown almost three
times (287%) higher bio-availability than
traditional Coenzyme product.
Research Fellows
Dr. Jimmy X. Wang, as a senior chemist and continuous entrepreneur his expertise includes 1) comprehensive knowledge, skills and experience in APIs, IPIs, formulations, analytic method validation development, animal and human clinical studies and protocol designs; 2) FDA compliance for DMF, DS, MF, OTC, ANDA, NDA regulatory affairs and GMP facility inspection, pre-audit, 3) solid & liquid dosage form development of specialty drug delivery system in the SEDDS technology field of formulation/process design, development, scale-up, optimization and process validation; and 4) managerial experience and leadership in R&D and operations. He successfully built up leading Nutraceutical Operations for the AHF companies 4 different locations and implemented the products development driven and cost-effective program. Dr. Wang successfully managed and supervised scientists to develop and design various products for multiple compounds and formulations including more than 50 new products approved and sold in the US market and more than 25 new products applications still pending for approvals and will being introduced to the global market in 2020.
Dr. Jimmy X. Wang obtained BS degree from Nanjing Normal University in 1982, respectively, followed by MS degree and Ph.D. degree in Chemistry from NYU Polytechnic University in New York in 1991. Before studied in US, he was an associate professor in the chemistry department in University China. After graduated from NYU Poly, he joined with DuPont Pharmaceuticals as a senior chemist and then worked with a FDA Regulatory consulting firm as a senior scientist. Dr. Wang is a continuous entrepreneur and founded the API company of MTC, Manufacturing company of AHF and Research Institute of SLS in Long Island. Dr. Wang has more than 10 patents and over hundreds of publications and presentations.
Dr. Ping "Peter" Gao, Ph.D.
Retired from Abbvie July, 2019 at the position of Center Director, Research Fellow, NCE-Formulation
27+ years technical service at Pharmaceutical companies including Upjohn, Pharmacia, Pfizer, Amgen and Abbott/Abbvie
Scientific and technical expertise: biopharmaceutics property assessment and preformulation for tox and FIH, formulation sciences and manufacturing processes, enabling drug delivery technologies for poorly soluble drugs, biorelevant in vitro assessment of formulation performance for BA and BE, quantitative in vitro-in vivo relationships and etc.
Inventor of formulation in marketed products: tipranavir (HIV) and dasabuvir (HCV) with high drug loading and excellent bioavailability
Inventor/co-inventor of 24 granted US/European patents and 25 patent applications. 65 publications including peer-reviewed journal articles/book chapters, guest editors for Mol. Pharm. and AAPS J. and 60+ invited presentations at NIH, FDA, national and international conferences, workshops and short courses, and universities.
Elected as Fellow of American Association of Pharmaceutical Scientists (AAPS) in 2009.
AAPS Physical Pharmacy & Biopharmaceutics (PPB) Section Chair/vice chair: 2012-2014
Education: Ph.D. degree in Analytical Chemistry, Purdue University, IN, 1988
Andrew Wood, MS is a career Formulation and Analytical Chemist with extensive experience in formulation design and product development of nutraceutical and pharmaceutical preparations. Andrew’s technical expertise and leadership has resulted in the release of hundreds of ingestible therapeutic products incorporating dry dose and liquid dose formulations. He has extensive experience implementing formulations into a vast range of delivery systems that include capsules, tablets, powder preparations, soft-gels, liquid capsules, dropper, multi serve liquids, and sachets. In his 15 years of experience, Andrew has developed a reputation of successfully leading the collective technical and operational processes leading to the development then release of stable, viable, and regulatory compliant therapeutic products for human applications. Andrew intends to apply his knowledge and capabilities to the American health formulations team to drive and guide the science, technology, and engineering systems ensuring all products released from the firm satisfy the highest standards. His participation in the collective R&D and manufacturing systems is enhanced by his desire to provide effective tools to physicians and supportive products to consumers to improve overall health and well-being. Optimizing product development is his goal but providing a means to support health is his passion.
Dr. Li Yao has a strong cGMP/cGLP knowledge with experience in implementing FDA regulations applicable to laboratories in pharmaceutical settings.
Quality Driven by Science & Technology
Cardiovascular Health
SEDDS CoQPure
Ingredients: Coenzyme-Q10.
Functions:
1. Helps cells produce more energy
2. Supports heart health
3. Acts as an antioxidant helping protect from free radicals
4. Helps reduce the signs of normal aging
5. Helps maintain normal blood pressure levels
6. Helps boost the immune system
7. Supports the nervous system
Applicable Population: Adults.
SEDDS KrilPure
Ingredients: Krill Oil.
Functions:
1. Supports overall health with Omega-3 fatty acids, DHA, EPA and powerful antioxidants.
2. Provides multi-system health benefits with much higher absorption than fish oil and without the aftertaste, side effects, or pollutants from fish sources
3. May be beneficial in the management of heart disease, high cholesterol, the high blood pressure, osteoarthritis,depression, and premenstrual syndrome (PMS)
Applicable Population: Adults.
Bone & Joint Health
SEDDS Super Joint
Ingredients: Glucosamine, Soybean isoflavone, Epimedium, Collagen, Chondroitin sulfate.
Functions:
1. Protects articular cartilage, synovial fluid, joints, soft tissue, and the bone joint connection
2. Provides a full range of nutrition to effectively remove harmful enzyme joint cavity, inhibit inflammation, and combine with proteins to form proteoglycans;
3. Stimulate the synthesis of new cartilage.
Applicable Population:
Patients with periarthritis of shoulder, Meniscus injuries, Patella Osteomalacia, Ankylosing spondylitis, External humeral epicondylitis (tennis elbow), Lumbar disc herniation, Osteoporosis, and Rheumatoid arthritis.
Brain Health
Super Brain
Ingredients: Galantamine, Calcium ion, Zinc ion, Ginkgo flavone glycoside, ADS PS phosphatidyl serine, Huperzine.
Functions:
1.Repair the cranial nerve -- Galantamine;
2. Stabilize brain function -- Calcium ion;
3. Stabilize Zinc ion -- Brain function;
4. Dredge the Cerebral blood vessel -- Ginkgo flavone glycoside;
5. Activate Brain cells -- ADS PS;
6. Protect the Cerebral nerve--Huperzine.
Applicable Population: Those with Alzheimer's disease, Parkinson syndrome, Cerebral infarction, or Chronic brain fatigue.
Eye Health
Eye Bright
Ingredients: Haematococcus extract, Safflower seed oil, Chrysanthemum extract, Blueberry extract.
Functions:
1. Protect eyesight, prevent blindness, glaucoma, cataract, retinal hemorrhage; Improvement of myopia, macular degeneration, diabetic retinopathy, retinitis pigmentosa and night blindness, etc--Blueberry;
2. Prevent aging macular degeneration, protect the retina from oxidative damage in the absorption of light--Chrysanthemum extract;
3. Supplement Linoleic acid(about 80%). The 50% of structural components of retinal neural cells is α-linolenic acid--Safflower seed oil;
4. Act as the strongest natual anti-oxidant,
cross the "blood retinal barrier", repair oxidative damage of any part of the eye, quickly improve eye diseases--Haematococcus pluvialis;
Applicable Population: A variety of eye discomfort health.
Skin, Nail & Hair Health
Biotin
Ingredients: Biotin, Folic acid.
Functions:
1. Provide the precursor of the synthesis of vitamin C;
2. Supplement the essential substances for normal metabolism of lipids and protein, help to promote body fat burning, maintain the normal female figure;
3. Promote the hair growth, help the lipid metabolism, enhance the immunity, protect the eyesight;
Applicable Population: Adult female.
Liver & Kidney Health
Super Liver
Ingredients: Thistle
Functions:
1. Improve the acute or chronic hepatitis, liver cirrhosis, fatty liver, metabolism of toxic liver injury, cholelithiasis, cholangitis and biliary duct inflammation around the liver, biliary inflammatory disease;
2. Improve the symptoms in patients with liver and some biochemical indices such as serum bilirubin, prothrombin, alanine transaminase.
Applicable Population:
Alcoholic hepatitis, viral hepatitis, toxic mushrooms, cancer.
Antioxidant
AstaPure
Ingredients: Haematococcus pluvialis (Astaxanthin), Gelatin, Glycerin.
Functions:
1. Act as the Antioxidant;
2. Scavenge the free radicals, prevent the tumor, improve the eye discomfort and immunity.
Applicable Population: Adults.
Resveratrol
Ingredients: SEDDS Resveratrol 100mg
Functions:
1. Strong antioxidant properties
2. Promote healthy cholesterol levels
3. Support heart health
Applicable Population: Adults.
Publications
1 .US Patent Series No. 60/607, 320. J Wang, P. Gao, GW Lu,: Self Emulsifying Composition For
Delivering Lipophilic Active Ingredients.
2. Chinese Patent ZL2005 8 0029556.2 王小曾 用自乳化制剂释放亲脂性辅酶CoQ10及其他膳食组份.
3. US 6,121,313. P.Gao and W.Morozowich. Pharmaceutical composition in a form of self-emulsifying formulation for acidic lipophilic compounds.
4. US 6,231,887 B1. P.Gao and W.Morozowich. Pharmaceutical composition for lipophilic compounds in a form of self- emulsifying formulation.
5.US 6,451,838 B1. M.W.Moon, W.Morozowich, P.Gao. 1-(pyrrolidin-1-ylmethyl)-3-(pyrrol-2-ylmethylidene)-2-indolinone derivatives.
6.US 6,482,848 B2. Prodrugs of 3-(pyrrol-2-yl-methylidene)-2-indolinone derivatives by M.W.Moon, W.Morozowich, P.Gao, M.Koenig.
7.US 6,531,139 B1. P.Gao, W.Morozowich. Self-emulsifying formulation for lipophilic compounds.
8.US 6,579,895 B2. A.Karim, A.M.Brugger, P.Gao, F.Hassan, and J.C.Forbes. Use of a celecoxib composition for fast pain relief
9.EP 0989 851 B1. W.Morozowich and P.Gao. Self-emulsifying formulation for acidic lipophilic compounds.
10.EP 0999 838 B1. W.Morozowich and P.Gao. Self-emulsifying formulation for lipophilic compounds.
11.WO 01/90068 A2. Mannich base prodrug of 3-(pyrrol-2-ylmethylidene)-2-indolinone derivatives by M.W.Moon, W.Morozowich, P.Gao, P.C.Tang.
12.WO 01/90104 A2. Prodrugs of 3-(pyrrol-2-yl-methylidene)-2-indolinone derivatives by M.W.Moon, W.Morozowich, P.Gao.
13.Yunguo Gong, Chaoneng Wu, Qinghe Xing, Xinzhi Zhao, Jun Zhu, Lin He. A two-method Meta analysis of Neuregulin 1(NRG1) association and heterogeneity in schizophrenia. Schizophrenia Research. 2009, 111 (1-3):109-114. (Research article, IF4.6, cited times 36)
14.Chaoneng Wu, Yunguo Gong, Jie Yuan, Hui Gong, Yunzeng Zou, Junbo Ge. Identification of shared genetic susceptibility locus for coronary artery disease, type 2 diabetes and obesity: a meta-analysis of genome-wide studies. Cardiovasc Diabetol. 2012, 14;11 (1):68-80. (Research article, co-first author, IF4.0, cited times 6)
